Suspension is defined as the heterogeneous mixture in which solute particles suspended throughout the bulk of the particles. The particle size is more than 100 nm. In suspension, particles don't pass through filter paper. Sand in water is an example of suspension.
Colloid is defined as a mixture (heterogeneous and homogeneous) in which one substance of dispersed insoluble particles get suspended throughout other substance. The particle size is 1 to 100 nm. In colloid, particles are small, thus pass through filter paper. The particles of air which is dispersed in solid stone is an example colloid.
Emulsion is a mixture of two or more substance which are immiscible in nature. It is a part of colloid. Milk is an example of emulsion.
Solution is a homogeneous mixture with clear or transparent appearance. The particle size in solution is
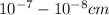 i.e. molecule in size. There is no effect of light occurs in the solution and solution can't filtered but can separated by the physical technique i.e. distillation.
i.e. molecule in size. There is no effect of light occurs in the solution and solution can't filtered but can separated by the physical technique i.e. distillation.