Answer : The percentage of protonated pentobarbitone is 20%
Explanation :
Let's say B represents pentobarbitone and BH⁺ represents protonated pentobarbitone.
The protonation reaction can be written as
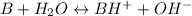
When the above reaction reaches equilibrium, we get a mixture of B and BH⁺.
This mixture contains a weak base pentobarbitone and its protonated form which is a conjugate acid. Therefore the mixture acts as a buffer.
The pH of a buffer is calculated using Henderson formula which is written below.
![pH = pka + log [(Base)/(acid)]](https://img.qammunity.org/2019/formulas/chemistry/college/3kpx1glcmr6tt7o7y03qvn5fiierjute1o.png)
We have acid = BH⁺
base = B
pH = 8.7
pka = 8.0
Let us plug in the above values in Henderson equation.
![8.7 = 8.0 + log [(B)/(BH^(+))]](https://img.qammunity.org/2019/formulas/chemistry/college/vcojceyc495qp1luupeddkz5tvxunolaor.png)
![8.7 - 8.0 = log [(B)/(BH^(+)) ]](https://img.qammunity.org/2019/formulas/chemistry/college/bxatzh0w3eit3nbho2bpfee1xdwc2z4rhy.png)
![0.7 = log [(B)/(BH^(+))]](https://img.qammunity.org/2019/formulas/chemistry/college/wbhfobyzar4cxc3v50rd98bz0y14euuanh.png)
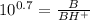
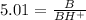
We want to find fraction of BH⁺ , which is written as BH⁺/B.
Therefore we will inverse the above equation.
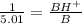
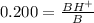
The percentage of protonated pentobarbitone is 0.200 x 100 = 20%