Answer: -
Molar mass of ferrous ammonium sulfate =392 g / mol
Mass of iron present in ferrous ammonium sulfate = 56 g/mol
Total mass required = 0.2 g
Total mass of iron Fe required = 10% of 0.2 g
=
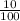 x0.2
x0.2
= 0.02 g
Only ferrous ammonium sulfate has iron. So all iron must be present only as ferrous ammonium sulfate.
56 g of iron is present in 392 g of ferrous ammonium sulfate
0.02 g of iron is present in
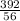 x 0.02
x 0.02
= 0.14 g
Mass of ammonium sulfate = 0.2 - 0.14 = 0.06 g
Thus the required composition must be 0.06 g ammonium sulfate and 0.14 g ferrous ammonium sulfate.