If the element has a atomic number of forty-four then the amount of neutrons would be "fifty-seven." This element is actually called Ruthenium and it has forty-four protons, and electrons, and it has fifty-seven neutrons and you can determine this by using this equation then solve:
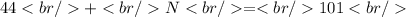
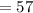
Hope this helps!