Answer: Molality = 2 mol/Kg or 2m
Molality is a property of a solution and is defined as the number of moles of solute per kilogram of solvent. The SI unit for molality is mol/kg or 'm'.
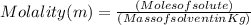
In our question , Solute is NaOH and solvent is water.
Moles of solute NaOH = 6 mol
Mass of solvent water = 3 kg
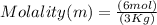
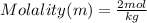
Molality = 2m