Density is defined as the ratio of mass to the volume.
Density =
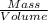 (1)
(1)
Mass of water = 10 grams
Mass of acetone = 10 grams
Density of water = 1
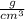
Density of acetone = 0.7857
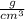
Put the value of density of water and its mass in equation (1)
1
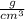 =
=
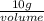
Volume of water = 10
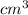
Put the value of density of acetone and its mass in equation (1)
0.7857
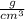 =
=
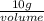
Volume of acetone = 12.72
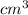
Thus, volume of acetone is more than volume of water because the density of acetone is lower.