Answer: 58%
Step-by-step explanation:
Formula used :
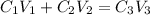
where,
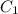 and
and
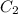 are the concentrations of first and second solution respectively
are the concentrations of first and second solution respectively
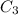 is the concentration of resulting solution
is the concentration of resulting solution
 and
and
 are the volumes of of first and second solution respectively
are the volumes of of first and second solution respectively
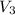 is the volume of resulting solution = 9 L
is the volume of resulting solution = 9 L
Now put all the given values in the above formula, we get the concentration of resulting solution.
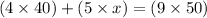
solving for x, we get
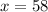
The concentration of the second solution= 58%.