Answer : There are
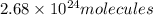 in 100 Lof potassium hydroxide(KOH) solution at stp.
in 100 Lof potassium hydroxide(KOH) solution at stp.
Explanation : To calculate the molecules we first need to find out the moles.
One mole of an ideal gas will occupy a volume of 22.4 liters at STP (Standard Temperature and Pressure, 0°C and one atmosphere pressure).
1 mole = 22.4 L
100 L =
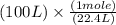
100 L =
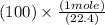
100 L = 4.46 mole potassium hydroxide(KOH)
Now , we need to convert 4.46 mole to molecules of KOH.
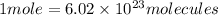
4.46 moles =
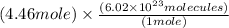
4.46 moles =
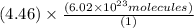
4.46 moles =
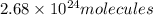
There are
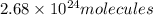 in 100 Lof potassium hydroxide(KOH) solution at STP.
in 100 Lof potassium hydroxide(KOH) solution at STP.