Answer:
![[OH^(-)]= 0.01M or 1.0* 10^(-2)M](https://img.qammunity.org/2019/formulas/chemistry/high-school/xolfys5ivdjz4yhfpe9cblkfy1g863g4f5.png)
![[H^(+)]= 1.0* 10^(-12)M](https://img.qammunity.org/2019/formulas/chemistry/high-school/r87oiekeb2urjwc9t52zbl7s92qwi5haga.png)
pH = 12
pOH = 2
Explanation: Calcium hydroxide (
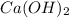 ) is a strong base that dissociates completely.
) is a strong base that dissociates completely.
Dissociation equation of Calcium hydroxide is :
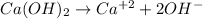
1. Concentration of [OH-]
1 mol
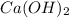 produces 2 mol OH- ions.
produces 2 mol OH- ions.
The given solution is 0.005M
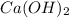 , then concentration of OH- would be twice the concentration of
, then concentration of OH- would be twice the concentration of
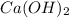
![[OH^(-)] = 0.005* 2](https://img.qammunity.org/2019/formulas/chemistry/high-school/twhkpx3h1igx1vk1vji323n59bggmkct2r.png) = 0.01M or
= 0.01M or
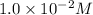
2.Concentration of [H+]
Concentration of [H+] can be calculated by the formula:
![[H^(+)] = (Kw)/([OH^(-)])](https://img.qammunity.org/2019/formulas/chemistry/high-school/p3l6tzregidd7nfdv9aetm5whiku0fycx9.png)
kw = ionic product of water and its values is
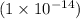
[OH-] = 0.01 M or
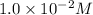
![[H^(+)] = ((1* 10^(-14)))/([OH^(-)])](https://img.qammunity.org/2019/formulas/chemistry/high-school/95pqvp44mwkfi3ovq4pzmhm2gem5jla9t9.png)
![[H^(+)] = ((1* 10^(-14)))/([0.01])](https://img.qammunity.org/2019/formulas/chemistry/high-school/dct7z3pylpoozq9xedvl9q1yudr42qaioe.png)
![[H^(+)] = 1.0* 10^(-12)M](https://img.qammunity.org/2019/formulas/chemistry/high-school/3m86p09ranee4dx33a2ey7sih873rqgmuj.png)
3. pH value
pH is calculated by the formula :
![pH = -log[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/zx3ffomnfpjqphyc55kzn36sue286kslxj.png)
![pH = -log[1.0* 10^(-12)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/g48vw8dpetquoy1lc8qx9f25ksrn3uvrpw.png)
pH = 12
4. pOH value
pOH is calculated by the formula : pOH = 14 - pH
pOH = 14 - 12
pOH = 2
pOH can also be calculated by using a different formula which is :
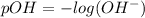
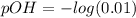
pOH = 2.