Answer : The concentration of Ca²⁺ ions is 2030 ppm CaCO₃ in the given solution.
Explanation :
Step 1 : Write chemical equation
The reaction of Ca ions and EDTA solution can be represented as follows.
![Ca^(2+) (aq) + EDTA^(4-) (aq) \rightarrow [Ca-EDTA]^(2-) (aq)](https://img.qammunity.org/2019/formulas/chemistry/college/849r9dxr6euzq5xrnazs7xqq0deddyehwb.png)
Step 2 : Find moles of EDTA
The molarity of EDTA solution is 0.05 mo/L
The volume of EDTA required for the reaction is 10.14 mL
The volume in liters =
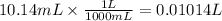
The moles of EDTA can be calculated using molarity formula which is given below.
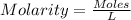
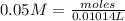
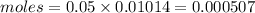
We have 0.000507 moles of EDTA.
Step 3 : Find moles of Ca ions.
The mole ratio of Ca ions and EDTA is 1 : 1.
We have 0.000507 mol EDTA.
Moles of Ca²⁺ ions =
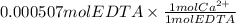
We have 0.000507 moles of Ca²⁺ ions.
Step 4 : Find mg of CaCO₃
The concentration of Ca²⁺ ions needs to be expressed as ppm CaCO₃.
We assume that all the Ca²⁺ ions come from CaCO₃.
So moles of CaCO₃ are same as moles of Ca²⁺ ions which is 0.000507.
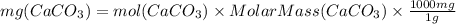
Molar mass of CaCO₃ is 100.1 g/mol
Let us plug in the values in above formula.
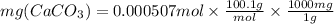
We have 50.75 mg CaCO₃
Step 5 : Find ppm using the formula
ppm CaCO₃ is calculated using following formula.
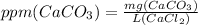
The volume of CaCl₂ solution is 25 mL which is 0.025 L.
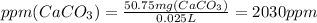
The concentration of Ca²⁺ ions is 2030 ppm CaCO₃ in the given solution.