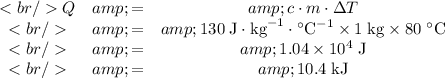
The specific heat of a material gives the energy it takes to increase the temperature of one unit mass of the material by one unit temperature. The SI unit for specific heat
 is therefore
is therefore
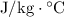 .
.
The formula relating the energy required to raise the temperature of
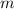 grams of a substance with specific energy
grams of a substance with specific energy
 by
by
 degrees is
degrees is
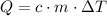
The question provides the following information:
Apply the formula: