Answer:
Volume = 24.9 cm³
Step-by-step explanation:
To calculate the volume of the piece of aluminum given its mass and density, we can use the following formula:
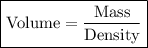 .
.
We are told that the piece of aluminum has a mass of 67.3 grams and that aluminum has a density of 2.70g/cm³. Using this information and the formula above, we can calculate the volume of the piece of aluminum:
Volume =
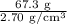
= 24.9 cm³
Therefore the volume of the piece of aluminum is 24.9 cm³.