Answer:- Density is 2.54 gram per mL.
Solution:-
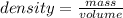
Mass of the marble ball is given as 9.513 g and it's volume is 3.75 mL.
To calculate the density of the marble ball let's plug in the values in the formula:
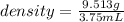
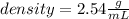
So, the density of the marble ball is 2.54 gram per mL.