Solution : Teachers percent error is 7.5%
Percent error can be calculated by using the percent error formula.
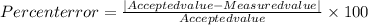
Accepted value is the true value.
Measured values is the calculated value.
In our question the True value (Accepted value) is given as 40 g/mol
Measured value (calculated value) is given as 37 g/mol
We will plug in the accepted value and measured value in the percent error formula and calculate the value of percent error.
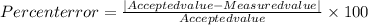
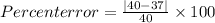
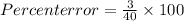
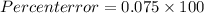
Percent error = 7.5 %