The amount of heat required for the sublimation of 40.0 g of solid dry ice CO₂ is 29.3 kJ.
One mole of CO₂ has a mass of 44.0095 g.
Calculate the number of moles n in 40.0 g of CO₂ .
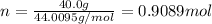
Heat of sublimation is the amount of heat required by 1 mole of a substance to convert itself from solid state to a vapor state at constant temperature and pressure.
1 mole of CO₂ requires 32.3 kJ of energy to sublimate.
Therefore, the heat required to sublimate 0.9089 mol of CO₂ is given by,
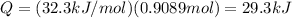
Thus, the heat required to sublimate 40.0 g of CO₂ is 29.3 kJ.