Answer:
Aluminum
Step-by-step explanation:
It is given that:
Mass of sugar = Mass of aluminum
The volume occupied by the two substances can be deduced from their densities.
Density of a substance is a constant and given by the ratio of its mass to volume.
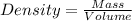
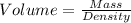
Density of aluminum = 2.7 g/cm3
Density of Sugar = 1.59 g/cm3
Since the masses are equal we have:
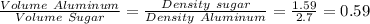
Volume of Aluminum = 0.59 (Volume of sugar)
Therefore, aluminum will require a container of smaller volume