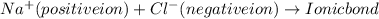Ionic bond is formed between positive and negative ions.
Positive ion is known as cation (electropositive metal)
Negative ion is known as anion ( electronegative non-metal)
The bond formed between a metal (positive ion) and non-metal (negative ions) is known as Ionic bond.
Ionic form is formed when one or more electrons from the valence shell of an atom (electropositive metal ) are completely transferred to the valence shell of another atom (electronegative non-metal). Ionic bonding is the complete transfer of valence electron(s) between oppositely charged atoms.
For example: Bond between NaCl(sodium chloride) is an ionic bond in which Na (sodium ) is positive ion(metal) which have +1 charge and Cl (chlorine) is negative ion(non-metal) which have -1 charge and the bond formed between Na+ and Cl- is ionic bond.