In the balanced equation, we see that the ratio of
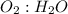 is 1:2 respectively. That being said, we can then set up a proportion to solve for the number of moles of water produced from 13.35 moles of oxygen:
is 1:2 respectively. That being said, we can then set up a proportion to solve for the number of moles of water produced from 13.35 moles of oxygen:
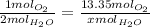
Then we cross multiply and solve for x:
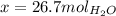
Therefore the answer is B) 26.70 mol.