We know that Carbon is going to have 6 electrons, and therefore it is more about knowing which orbitals these electrons are in and how many electrons each orbital can hold. Therefore the electron configuration of Carbon is:
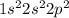
or, in short-hand, you can write the noble gas prior to the row that the element is in, and that assumes the electron configuration of that noble gas to be written there and then continue with the electrons in that row. So in this case, we could also write:
![[He]2s^(2)2p^(2)](https://img.qammunity.org/2019/formulas/chemistry/middle-school/zm2qw1usa1yp2nal9ljopmsrq8mo21yydy.png)