NH3–NH4Cl is a/an Buffer that helps regulate the pH of a system.
A buffer solution ( pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its salt formed from conjugate base, or weak base and its salt formed from conjugate acid.
Acidic Buffer : Consisting of a mixture of a weak acid and its salt formed from conjugate base.
Basic Buffer : Consisting of a mixture of a weak base and its salt formed from conjugate acid.
The given solution is NH3-NH4Cl is Basic buffer which is composed of weak base(NH3 ammonia) and a salt(NH4Cl ammonium chloride) formed from its conjugate acid(HCl hydrochloric acid).

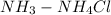 composed of weak base and its salt and is considered as Basic Buffer.
composed of weak base and its salt and is considered as Basic Buffer.