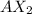Alkaline earth metal are the elements present in II group in the periodic table and are known as 'Metals' and have a charge of +2.
Alkaline earth metals - Be , Mg Ca, Sr , Ba, Ra
Halogens are present in VII A group in the periodic table and are 'Non-metals' and have a charge of -1.
Halogens - F, Cl, Br, I, At
When Alkaline earth metal (metals) combine with Halogens (non-metals) the compound formed will be ionic compound and the formula of the compound will be based on the charges of the element.
When we write the formula of the ionic compound the charges of the elements get criss crossed.
For example - Mg (Alkaline earth metal) have a charge of +2 and Cl (Halogen) have a charge of -1 and when they combine to form the formula their charges get criss crossed and we will get
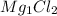 or
or
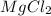
When an alkaline earth metal, A, reacts with a halogen, X, the formula of the Ionic compound formed should be