Answer:- 23.8 kJ of heat is required to raise the temperature of water.
Solution:- We have been given with grams of water, it's initial and final temperature and asked to calculate the heat required to raise it's temperature. Here, lower degree C water is changing into higher degree C water. So, the formula used to calculate the heat is:
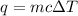
Where, q is the heat energy, m is mass, c is specific heat of water and delta T is change in temperature.
m = 100 grams
 = 82 - 25 = 57 degree C
= 82 - 25 = 57 degree C
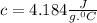
q=?
Let's plug in the values in the formula and do the calculations:
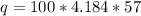
q = 23848.8 J or 23.8 kJ
So, 23.8 kJ of heat is required to raise the temperature of 100 g of water from 25 to 82 degree C.