Answer: The boiling point of the liquid is 47.368°C.
Step-by-step explanation: The percent error is a tool used to determine the precision of the calculations.
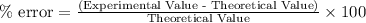
Here, we need to calculate the Theoretical Value,
Experimental value = 45°C
% error = -5.0%
Let theoretical value be "x"
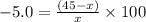

The correct boiling point of the unknown liquid is 47.368°C