Answer:- An empirical formula of adrenaline is
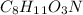 .
.
Solution:- Convert given percentages to moles and then calculate the mol ratio to get the simplest whole number ratio of moles of atoms which is the empirical formula of the compound.
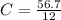 = 4.725 mol
= 4.725 mol
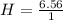 = 6.56 mol
= 6.56 mol
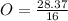 = 1.773 mol
= 1.773 mol
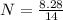 = 0.591 mol
= 0.591 mol
let's calculate the mol ratio now. To calculate the mole ratio, we divide the moles of each by moleso f N as it's the least one:
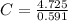 = 8
= 8
 = 11
= 11
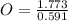 = 3
= 3
 = 1
= 1
So, an empirical formula of adrenaline is
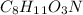 .
.