pH = 2.1
Let
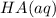 resembles the acid; as a weak acid (a small value of
resembles the acid; as a weak acid (a small value of
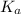 )
)
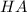 would partially dissociate to produce protons
would partially dissociate to produce protons
 and
and
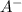 , its conjugate base. Let the final proton concentration (i.e.,
, its conjugate base. Let the final proton concentration (i.e.,
![[H^(+)]](https://img.qammunity.org/2019/formulas/chemistry/college/75vk4lm3dg3i0qv728qhnrrb5qib1loutc.png) ) be
) be
 . (Apparently
. (Apparently
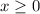 ) Construct the following RICE table:
) Construct the following RICE table:
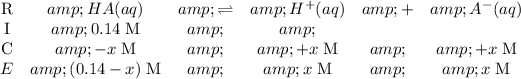
By definition, (all concentrations are under equilibrium condition)
![\left\begin{array}{ccc}K_(a)&=&[H^(+)] \cdot [A^(-)] / [HA]\\&=&x^(2) /(0.14 - x)\end{array}\right](https://img.qammunity.org/2019/formulas/chemistry/college/fb4ao1t1zeu9uphahsr769zoxqaj2kijkp.png)
It is given that
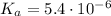
Equating and simplifying the two expressions gives a quadratic equation; solve the equation for
 gives:
gives:
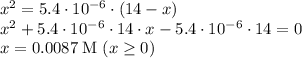
The pH of a solutions equals the opposite of the logarithm of its proton concentration to base 10; thus for this particular solution
![\text{pH} = -\text{ln(}[H^(+)]\text{)} / \text{ln(}10\text{)} = 2.1](https://img.qammunity.org/2019/formulas/chemistry/college/13of4m396yczdp2fn8t7tlr8uuk39bouf9.png)