Step-by-step explanation:
An oxygen atom is represented by the symbol O. And, the number placed in front of the atom or at the bottom left corner of an atom shows how many atoms are actually present.
For example,
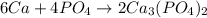
Here, on the reactant side number of Ca atoms are 6, P atoms are 4 and O atoms are 16. On the product side, number of Ca atoms are 6, P atoms are 4 and O atoms are 16.
Thus, we can conclude that the number of oxygen atoms on product side are 16.