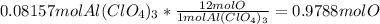Aluminum chlorate ionizes as follows,
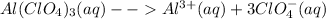
1 mol Aluminum chlorate ionizes to give 1 mole Aluminum ion and 3 moles of chlorate ion. Each mole aluminum ion has 4 * 3 = 12 moles of Oxygen atom.
Moles of
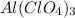 = 0.08157 mol
= 0.08157 mol
a) Moles of
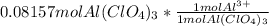 =
=
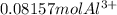
b)Moles of
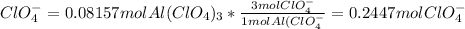
c) Moles of O atom =