Hello!
We have the following data:
P (Pressure) =? (atm)
V (volume) = 20.0 L
n (mol number) = 0.896 mol
R (gas constant) = 0.082 atm.L / mol.K
T (temperature) = 42 °C (in SI the temperature is given in Kelvin, needs to turn into Kelvin)
TK = TºC + 273.15
TK = 42 + 273.15
TK = 315.15
------------------
Applying the data to the Clapeyron equation, we have:
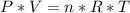
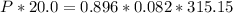
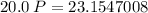
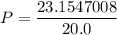

I Hope this helps, greetings ... DexteR!