Answer: Option (B) is the correct answer.
Step-by-step explanation:
- An ionic bond is formed by the sharing of electrons between two chemically combining atoms.
In an ionic bond, there occurs attraction between oppositely charged ions due to which there occurs strong forces of attraction between them. Therefore, ionic bonds are the strongest bonds.
- A polar covalent bond is formed due to unequal sharing of electrons between the combining atoms.
For example,
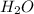 is a polar covalent compound. Partial opposite charges tend to develop on the atoms of a polar covalent compound.
is a polar covalent compound. Partial opposite charges tend to develop on the atoms of a polar covalent compound.
- A non-polar covalent bond is formed due to equal sharing of electrons between the combining atoms.
For example,
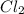 is a non-polar covalent molecule. No partial charges will be there on the atoms of a non-polar covalent molecule.
is a non-polar covalent molecule. No partial charges will be there on the atoms of a non-polar covalent molecule.
- A hydrogen bond is defined as the bond formed between a hydrogen atom and an electronegative atom.
For example, in HCl compound there occurs hydrogen bonding.
In this type of bond, dipole-dipole attractive interactions tend to take place. And, strength of hydrogen bonds is very weak.
Thus, we can conclude that given bond types are arranged in order of increasing strength as follows.
Hydrogen bonds < non-polar covalent bonds < polar covalent bonds < ionic bonds