% mass of a solution is the mass of the solute present per 100 g of solution. It can be calculated using the formula,
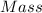
Mass of solute = 4.92 g
Mass of solvent, water = 347 g
Total mass of solution = 347 g + 4.92 g = 351.92 g
Mass % of the solute =
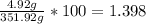
Therefore, the weight/weight % = 1.398 %