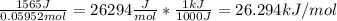The chemical equation representing the neutralization reaction between HCl and
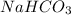 is,
is,
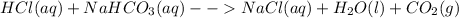
Given mass of
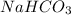 = 5g
= 5g
Moles of
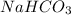 =
=
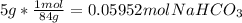
Volume of HCl solution =
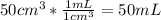
Assuming the density of solution to be 1.0 g/mL
Mass of HCl solution = 50 g
Total mass of solution = 50 g+ 5 g = 55 g
Calculating the heat of neutralization:
Q = m C ΔT
m is mass of solution = 55 g
C is the specific heat capacity of the solution = 4.184
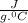
ΔT = Temperature difference = 6.8 K = (6.8 -273 ) C = -266.2
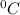
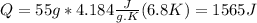
Enthalpy of neutralization per mole of
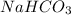
=