Answer:- density of the liquid is 0.21 gram per mL.
Solution:-
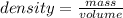
From given data, mass of cylinder = 10.4 g
mass of cylinder along with liquid = 11.7 g
So, mass of liquid = 11.7 g - 10.4 g = 1.3 g
Volume of liquid = 6.2 mL
Let's divide the mass by the volume to get the density of the liquid:
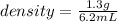
density =
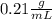
So, the density of the liquid is 0.21 gram per mL.