Answer:
The correct answer is: 'The helium at 15 °C has a higher average kinetic energy than the sample at 215 K'.
Step-by-step explanation:
Average kinetic energy of the helium atom at 15 °C .
Temperature of the gas = T = 15 °C = 288.15 K
Average kinetic energy is determined by formula:
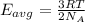
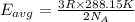
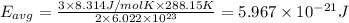
Average kinetic energy of the helium atom at 215 K.
Temperature of the gas = T' = 215 K
Average kinetic energy is determined by formula:
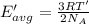
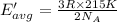
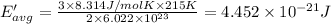
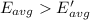
The helium at 15 °C has a higher average kinetic energy than the sample at 215 K.Hence, it is the correct option.