Answer: Kinetic Energy of the atoms also increases.
Explanation: We are given that the temperature of the gas increases.
Relation between kinetic energy and temperature follows:
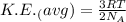
where, K = Average Kinetic energy
R = Gas constant
T = Temperature
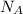 = Avogadro's number
= Avogadro's number
As seen from the relation above, the Kinetic energy of the gas is directly proportional to the temperature, hence as the temperature increases, kinetic energy of the atom also increases.