Answer : The partial pressure of
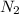 in the mixture is, 1.224 atm
in the mixture is, 1.224 atm
Solution :
According to the Dalton's law, the total pressure of the gas is equal to the sum of the partial pressure of the mixture of gasses.

where,
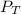 = total partial pressure of
= total partial pressure of
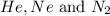 = 1.348 atm
= 1.348 atm
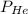 = partial pressure of helium
= partial pressure of helium
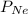 = partial pressure of neon
= partial pressure of neon
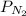 = partial pressure of nitrogen
= partial pressure of nitrogen
As we are given that,
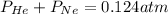
Now put all the given values in above expression, we get the partial pressure of the nitrogen gas in the mixture.
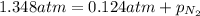
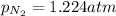
Therefore, the partial pressure of
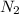 in the mixture is, 66 Kpa
in the mixture is, 66 Kpa