Answer:- The right choice is A.
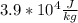 .
.
Solution:- Latent heat of fusion means the heat required to melt the solid at constant temperature means there is no change in temperature only the solid changes to liquid. So, it is a solid to liquid phase change.
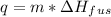
where q is the heat required to convert solid to liquid, m is the mass and
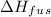 is the latent heat of fusion.
is the latent heat of fusion.
From given info, 550 kJ that is 550000 J of heat is required to melt 14 kg of solid at 262K temperature. Let's rearrange the equation for latent heat of fusion and plug in the values in it.
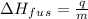
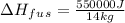
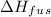 =
=
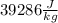
If we round this value to two sig figs and write in scientific notations then it becomes
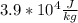 .
.
So, the right choice is A.
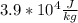 .
.