Answer:- The hydroxide ion concentration of the solution is
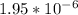 .
.
Solution:- The formula used to calculate pOH from hydroxide ion is:
![pOH=-log[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/bb6nd3dwmelw6hrf97fgv6xj1ptgdgg61f.png)
When pOH is given and we are asked to calculate hydroxide ion concentration then we multiply both sides by negative sign and take antilog and what we get on doing this is:
![[OH^-]=10^-^p^O^H](https://img.qammunity.org/2019/formulas/chemistry/middle-school/nun3zxba9m8mxydqybxfnovd44lqcbhnoq.png)
pOH is given as 5.71 and we are asked to calculate hydrogen ion concentration. Let's plug in the given value in the formula:
![[OH^-]=10^-^5^.^7^1](https://img.qammunity.org/2019/formulas/chemistry/middle-school/w3cdq4db8b8knpf1eew85qr8y4bjioyy2e.png)
![[OH^-]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/dr3vksfxq7ot5rvw2mjmnw96oc1xtussa2.png) = 0.00000195 or
= 0.00000195 or
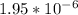
So, the hydroxide ion concentration of the solution is
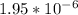 .
.