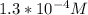pOH is the negative logarithm of hydroxide ion concentration in the solution. Lower pOH values, less than 7 denote that the solution is basic, higher pOH value, greater than 7 indicate an acidic solution.
![pOH = - log[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dmcfiblpncm1okj7xmp2tk3qzo0clgz060.png)
![[OH^(-)] = 10^(-pOH)](https://img.qammunity.org/2019/formulas/chemistry/high-school/rfpxx0834x11708v8g4yaew0d9q7qrfqg1.png)
Given the pOH of solution is 3.90.
Calculating the concentration of hydroxide ions,
![[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dbzut40quqceef230a4l1arx1nzd8511gj.png) from pOH:
from pOH:
![pOH = - log[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/dmcfiblpncm1okj7xmp2tk3qzo0clgz060.png)
![3.90 = - log[OH^(-)]](https://img.qammunity.org/2019/formulas/chemistry/high-school/76h9fn8bjmzny17tpcb6jm0g4n314dor63.png)
![[OH^(-)] = 10^(-pOH)](https://img.qammunity.org/2019/formulas/chemistry/high-school/rfpxx0834x11708v8g4yaew0d9q7qrfqg1.png)
![[OH^(-)] = 10^(-3.90)](https://img.qammunity.org/2019/formulas/chemistry/high-school/lcjdbgu4hmvqv1wiffh6q5g41ieyt4ublc.png)
=
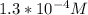
Therefore the hydroxide ion concentration of the solution will be