Answer: The correct answer is Option D.
Step-by-step explanation:
Valence electrons are defined as the electrons which are present in the outermost shell of an atom. Outermost shell has the highest value of 'n' that is principal quantum number.
Sulfur is the 16th element of the periodic table having 16 electrons. The electronic configuration of this element is:
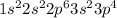
The number of electrons in first shell (n = 1) : 2
The number of electrons in second shell (n = 2) : (2 + 6) = 8
The number of electrons in third shell, outermost shell (n = 3) : (2 + 4) = 6
The number of valence electrons in sulfur atom are 6
Hence, the correct answer is Option D.