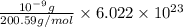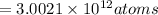Answer:
a) Mass of
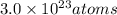 of mercury is 99.91 g.
of mercury is 99.91 g.
b)
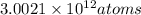 in 1 nano gram of mercury.
in 1 nano gram of mercury.
Step-by-step explanation:
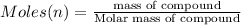
Number of molecules or number of atoms:

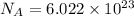 atoms or molecules
atoms or molecules
Mercury has an atomic mass of 200.59 amu = 200.59 g/mol
Number of mercury atoms =
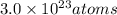
Moles of Mercury :
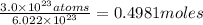
Mass of 0.4981 moles of mercury:
0.4981 moles × 200.59 amu = 99.91 g
Mass of
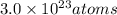 of mercury is 99.91 g.
of mercury is 99.91 g.
b) Mass of mercury = 1 nano gram =
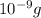
Moles of mercury:
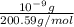
Number of atoms of mercury in 1 nano gram:
: