Answer:
C. Yes. The density equals that of pure silver.
Step-by-step explanation:
Yes the unknown metal is pure silver.
Density is the ratio of the mass of the substance to its volume. Density is directly proportional to that if mass and inversely proportional to that of volume.
Density =
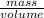 =
=
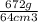 cm³
cm³
= 10.5 g/cm³
Here the density of the unknown metal is the same as that of silver.