Step-by-step explanation:
Atomic mass of a substance means the sum of total number of protons and neutrons present in an atom.
Whereas atomic number means the total number of protons present in an atom.
For example, in
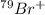 atomic mass is 79 and it is known that atomic number of Br is 35.
atomic mass is 79 and it is known that atomic number of Br is 35.
Hence, total number of protons, electrons and neutrons present in
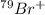 are as follows.
are as follows.
Atomic mass = No. of protons + No. of neutrons
79 = 35 + No. of neutrons
No. of neutrons = 79 - 35 = 44
When an atom is neutral then number of protons equal number of electrons. And, a positive charge means loss of an electrons.
So, number of electrons present in
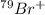 is 35 - 1 = 34.
is 35 - 1 = 34.
Thus, we can conclude that in
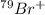 number of protons are 35, number of neutrons are 44 and number of electrons are 34.
number of protons are 35, number of neutrons are 44 and number of electrons are 34.