Answer:- The substance is Propane and right choice is C.
Solution:- 10140000 J of heat is used to boil 28.47 kg at 298 K. Here, the important thing is, the liquid is changing to vapor and the temperature is not changing means it's a phase change. So, the formula used for this is:
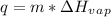
where, q is the heat energy, m is mass and
 is the enthalpy of vaporization.
is the enthalpy of vaporization.
Let's rearrange the formula for
 as:
as:
 =
=

Let's plug in the values:
 =
=
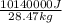
 =
=
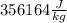
If we round this then it is
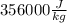 . Hence, the right choice is C. propane.
. Hence, the right choice is C. propane.