Heat require to boil 750 g water can be calculated from the formula:
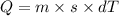
Here, Q is the heat
m, is the mass
s is the specific heat
dT is the temperature difference
Here ,
m, is 750 g
s of water is 4.2 cal g⁻¹ K⁻
dT , as water is boiled from 25 to 100 C
Q=
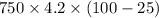
= 236250 J
Assuming if input power is 1000 W then time required is:
Q=
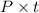
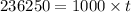
t = 236.25 s
Thus time required to boil 750 g of water will be 236.25 s