The balanced chemical equation between iron and oxygen to produce iron (III) oxide is,
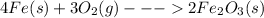
Mass of Fe = 227.8 g
Moles of Fe =
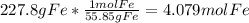
Mass of oxygen = 128 g
Moles of
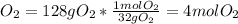
Calculating the limiting reactant: The reactant that produces the least amount of product will be the limiting reactant.
Mass of iron (III) oxide produced from Iron =
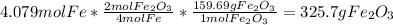
Mass of iron (III) oxide produced from oxygen=
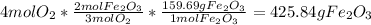
Iron (Fe) produces the least amount of the product iron (III) oxide. So, Fe is the limiting reactant.