Answer:- No, the company would not be able to full fill the order.
Solution:- The balanced equation for the formation of ammonia by the reaction of hydrogen with nitrogen is:
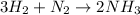
We have been given with 22000 grams of nitrogen and 1000 grams of hydrogen. Let's convert grams of each to moles:
moles of nitrogen =
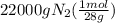
moles of nitrogen = 785.71 mol
moles of hydrogen =
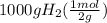
moles of hydrogen = 500 mol
From balanced equation 3 moles of hydrogen react with 1 mol of nitrogen. Let's calculate how many moles of nitrogen would react with 500 mol of hydrogen:
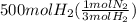
=
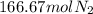
166.67 moles of nitrogen are required and 785.71 moles of it are available. It means nitrogen is in excess and hydrogen is limiting. Product yield depends on limiting reactant. So, let's calculate the amount of ammonia formed from moles of hydrogen as:
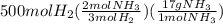
= 5666.67g of ammonia
let's convert the grams of ammonia to number of batches as:
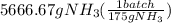
= 32 batches
Company needs to make 70 batches of ammonia but from given amounts of hydrogen and nitrogen only 32 batches could be made. It means the company would not be able to full fill the order.