Answer: The atomic number of the new element increases by 1 unit.
Explanation:
Beta-decay: In this process, a neutron gets converted into a proton and an electron releasing a beta-particle. The beta particle released is basically a electron with -1 charge and no mass.
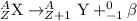
General representation of an element is given as:
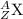
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element