The equation that relates both energy and wavelength is:
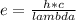
where e is the energy and lambda is the wavelength.
Therefore, as we can see from this equation, the energy of an electromagnetic wave is inversely related to the wavelength of the electromagnetic wave.