A nuclear reaction involves the reaction of nuclei of atoms. This can be expressed in the form of nuclear equation in which the reactant nuclei are represented by their respective symbols carrying two numbers, the mass number denoted by A present at the top left and the atomic number (Z) written at the bottom left of the symbol of the element's nucleus.
The given nuclear reaction is the fusion of two isotopes of hydrogen, H-2 to form Helium-3 nucleus:
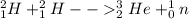
This reaction produces a single neutron.