Answer:- Volume of the 5.0 carat diamond is
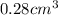 .
.
Solution:- It is a unit conversion problem. Need to convert carat to grams and then using grams and density the volume could easily be calculated.
We have 5.0 carat diamond and asked to calculate it's volume. Density of diamond is given as
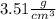 and 1 carat = 0.200 g. Let's do this step by step:
and 1 carat = 0.200 g. Let's do this step by step:
First step is the conversion of carat to grams:
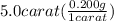 = 1.0 g
= 1.0 g
In second step, grams are converted to volume using the given density as:
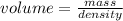
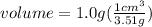
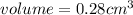
So, the volume of the 5.0 carat diamond is
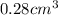 .
.